What a brain looks like: EM
Previous in series:
What a brain looks like: the whole brain
What a brain looks like: sections
The structure of a brain
What a brain looks like: stains
Background
The stains showed in the last post are used with light microscopes—not light, as in the opposite of heavy, but light, as in the stuff that hits your eyes. When preparing a sample to be looked at with an electron microscope (“EM”), there’s two stains that are almost always used: uranium1 and osmium. Uranium stains the background, increasing the contrast, and osmium binds with the membranes.
I can’t think of a heading title…moving on.
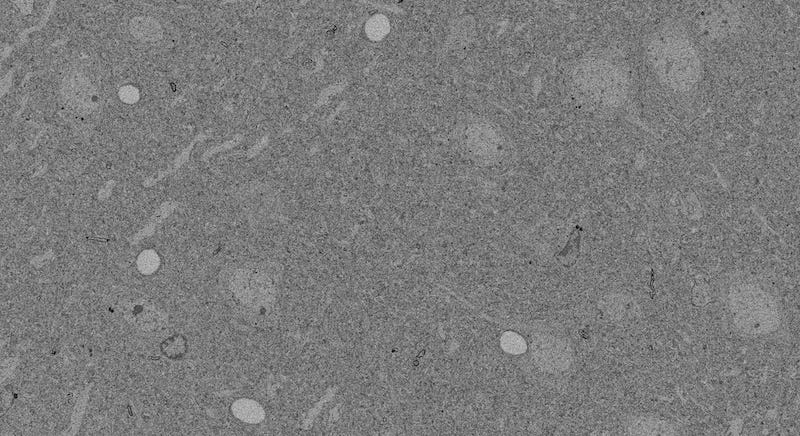
At second glance—that is, after you squint hard and realize there’s more going on than just a mass of boring grey—it looks pretty similar to the stains from the last post. There’s capillary holes, nuclei, a vague indication of some cell bodies around the nuclei, and a background sea of indistinguishable stuff.
You might wonder why the boundaries around the cell don’t stick out more, since osmium binds with cell membranes. You’ll see why, in a moment.
But first, here’s a picture gratuitously included because it looks cool.
Now let’s zoom in.

You’ve got the nucleus up top, and a cell body around it. The background sea of processes is starting to take on some form now.
And that’s it: the reason the membrane around the cell body doesn’t stick out, is because everything is cell membranes at this level. Every tiny process has its own wall, identical in type to the one around the cell body. If anything, the membrane around the nucleus sticks out, because that’s a double-layer with some other stuff stuck in it.
Let’s zoom in again, because electron microscopes can go a lot smaller than light microscopes.
This is what processes look like: weird, jumbled, twisted shapes.
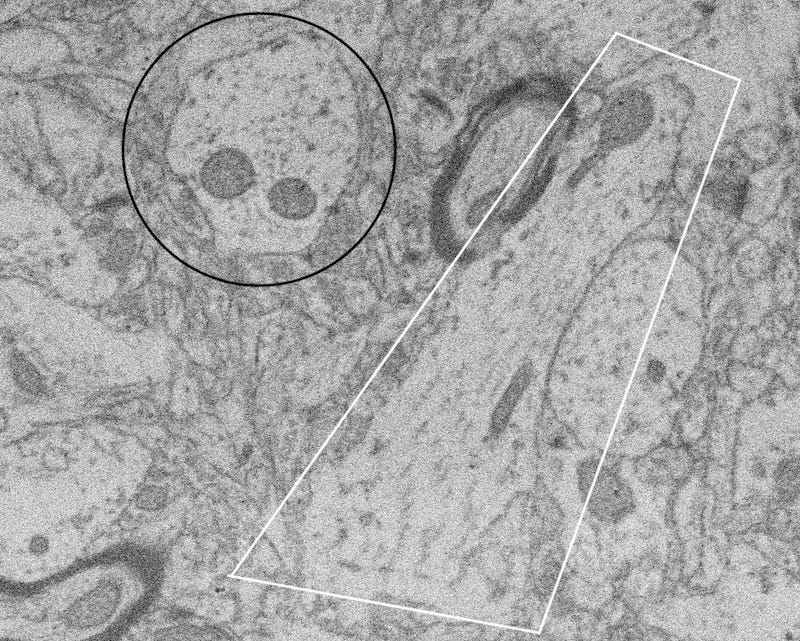
Let me help identify what you’re seeing, one step at a time, starting with those darkish blobs.
Mitochondria are those little things in cells that turn glucose and oxygen into ATP, the chemical that your body uses as little floating batteries. Processes need energy too, so they have mitochondria in them.
Microtubules, aka microfilaments, aka actin fibers: these are likes bones for cells. Also like ladders, because sometimes proteins or nutrients or whatever use the actin fibers to get to wherever they’re supposed to be. Or maybe like those moving walkways at airports, I’m not sure.
Circled in black is a process that you’re viewing a neat cross-section of, with two mitochondria. You can tell you’re viewing it head-on because the overall shape is roughly circular, i.e. condensed, and because the actin fibers look like (fuzzy) dots, meaning they’re coming straight out of the page at you.
The process in white, which also has two-ish2 mitochondria, is running sideways to your view. You can tell this because it has an elongated shape instead of a shortened one, and because the actin fibers look like fuzzy lines instead of fuzzy dots. The process either curves enough to move out of the plane of view on the ends, or, more likely, it’s not a perfectly perpendicular view; it could be at, say, 45°.
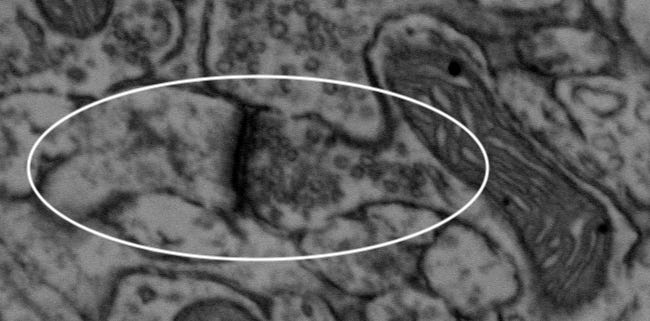
This is a synapse. See that slew of tiny empty-looking circles in there? Those are vesicles, and they’re found on the pre-synaptic side of the synapse. You can kinda-almost view the tiny cleft inbetween. What really marks the synapse is that dark bit, which is called the post-synaptic density, or PSD.
Once you know how to identify a synapse, you can zoom back out a bit and find more.
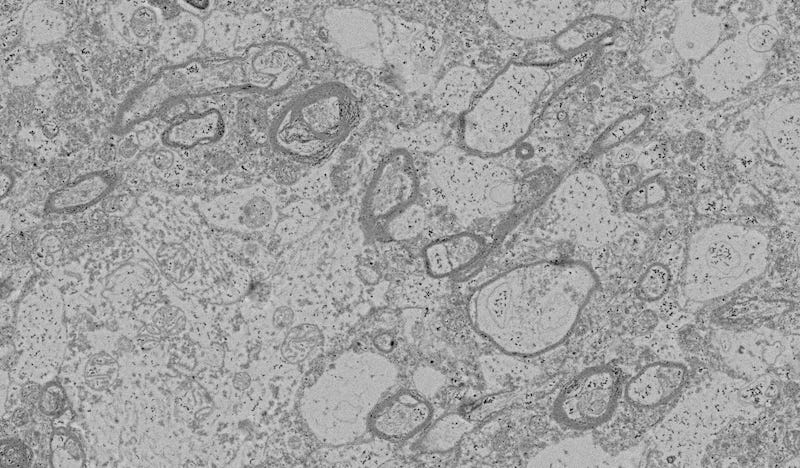
The regions I’ve been showing you so far are (mostly) neuropil3—areas with lots of processes and synapses and few or no cell bodies or myelin.
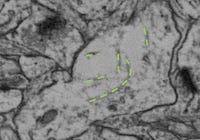
Myelin, on EM, shows up as dark black boundaries around the axons4. (Can you spot the synapse on this picture?)
When it comes to EM, what we’re interested in is the traceability of all the processes. We want to be able to build a complete reconstruction—in theory, not in practice; it would take too long for the whole brain—of where every process and synapse is, in 3D. That means we need to be able to see all the membranes well enough to clearly delineate what belongs to one process versus another, and we especially need to be able to see the synapses where the connections between neurons happen.
So far, the images I’ve shown you have good intrinsic traceability. I might not be able to trace them completely from these images—the lightest one has poor staining, several of them need better focusing by the EM, and in a few places, the 3D context might be required to put things together properly—but those are issues with how the sample was handled after preservation, not issues with the preservation itself.
Here’s an image that is not traceable:
There’s vast swaths that have been washed out, with no hope of finding most membranes. That’s not an issue with staining or imaging; the damage is there because the tissue wasn’t fixed well enough in the first place.
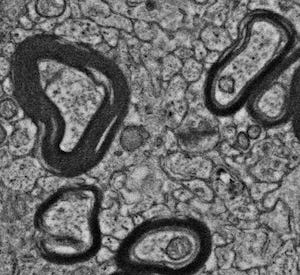
When myelin starts to degrade, it unravels, forming what are called myelin figures:
That can make tracing more difficult, but not necessarily impossible5; each myelinated process can still be distinguished from each other.
If the myelin figures are outward-focused whorls like this, they can potentially interfere with tracing nearby unmyelinated processes.
Especially if they get to the point of looking like this confusing mess, which I’m mostly sure is severely unraveled myelin:
To wrap this up, now that you can appreciate this more, you might want to go back and watch the 3D reconstruction on this video (4:10 to at least 5:20):
Or more specifically, uranyl acetate, but it’s still a radioactive yellow powder that can kill you. And Osmium might be the more dangerous of the two.
I do not know what’s going on with that mitochondria that looks like a maraca.
Not to be confused with neutrophils, which are a type of white blood cell.
Technically, osmium loves myelin, which is made of the same stuff as cell membranes, so when the electrons in the EM ping off the osmium, it returns a positive signal, which shows up as white. But biologists prefer to use black-white inverted images like the ones I’m showing here, so white is reversed to black.
By the time you see this post, we should be having a call with our preservation/tracing expert about whether one particular similar-ish-but-better-looking experiment counts as good enough or not.